| What? |
 |
 |
 |
| Written by DPR Biocides - Anses |
|
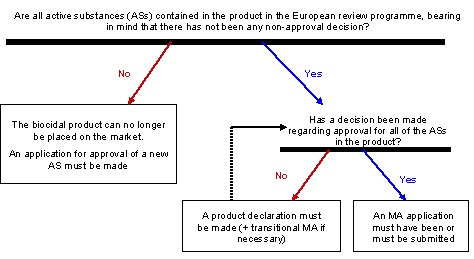
Any biocidal product placed on the French market must:
Moreover, Article 95 specifies that since 1 September 2015, any biocidal product is made available on the market only on condition that the supplier of the product or substance is included on a list held by ECHA
NB: Registration of a product in Simmbad or Synapse does not qualify as an MA application. These are only declarative databases. |
| Last Updated on Friday, 15 April 2022 09:30 |